Main listed companies in heart valve industry: Bai Ren Medical (688198.SH), Blue Sail Medical (002382.SZ), Lepu Medical (300003.SZ), Xintai Medical (02291.HK), Qiming Medical (02500.HK), Pejia Medical (09996.HK), Jianshi Technology (09877.HK), Microinvasive Medical (00853.H) K), Heart Medical (02160.HK), etc
Core data of this paper: China's heart valve industry chain panorama, China's heart valve enterprises regional competition
The upstream of the industrial chain is the supply of raw materials, and the downstream is the hospital
Upstream participants in the heart valve industry chain are raw material suppliers, including biological materials, chemical raw materials, steel and non-ferrous metals. Among them, biological materials include bovine pericardium, bovine jugular vein, and pig aortic valve. For midstream heart valve replacement and repair solution providers, animal tissue procurement amount is small; There are many kinds of chemical reagents purchased, but the overall amount of chemical reagents purchased is small; The purchase of packaging materials mainly includes packaging sponge boxes, outer packaging bags, outer packaging boxes, sealing labels, color pages, product manuals, cartons, etc., the relative amount is large. The middle part of the heart valve industry is mainly heart valve replacement and repair solution providers, including TAVR/TAVI manufacturers, TPVr/TPVR manufacturers, TMVr/TMVR manufacturers and TTVr/TTVR manufacturers. Downstream applications involve hospitals and pharmaceutical distribution enterprises.
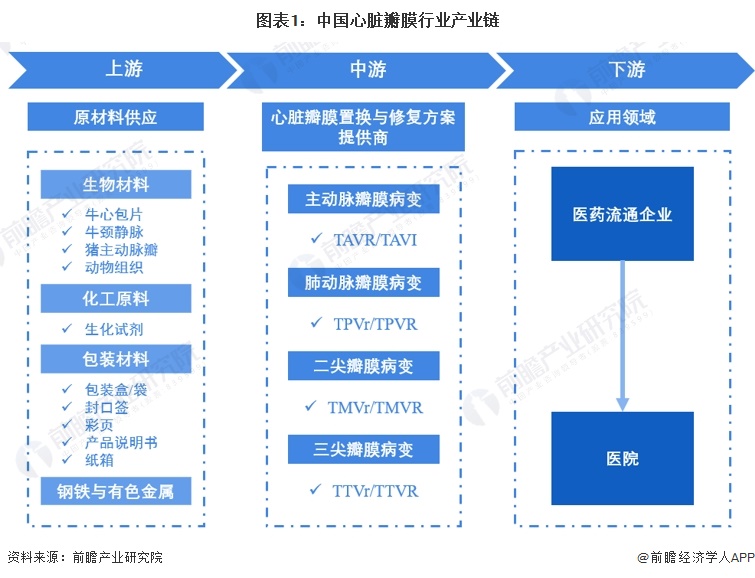
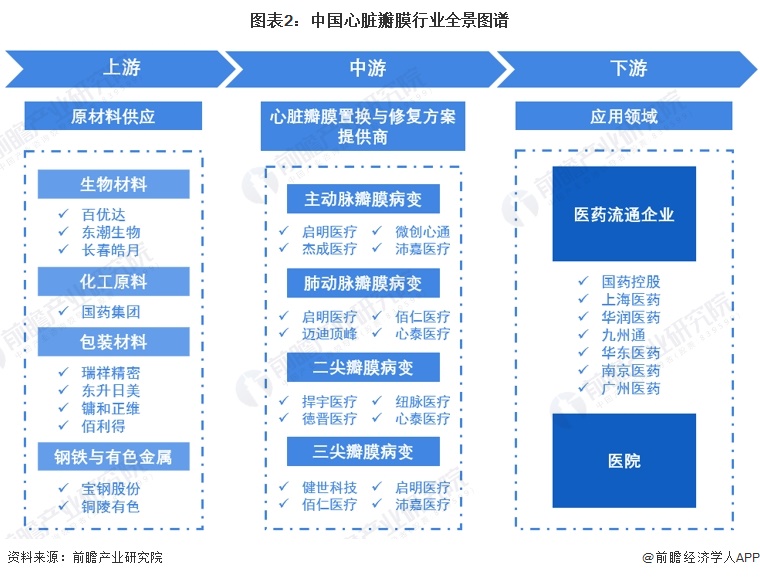
Among the participants in the heart valve industry chain, the upstream biomaterial manufacturers include amino acid Baiyouda, Dongchao Biology, etc., chemical raw material manufacturers Sinopharm Group, packaging material manufacturers, steel and non-ferrous metal manufacturers; Middle heart valve replacement and repair solution providers include Qiming Medical, Bairen Medical, Microinvasive Heart Tong, Xintai Medical, etc. Downstream pharmaceutical circulation enterprises mainly include Sinopharm Holdings, Shanghai Pharmaceutical, etc., and hospitals are the main application scenarios of heart valve replacement and repair programs.
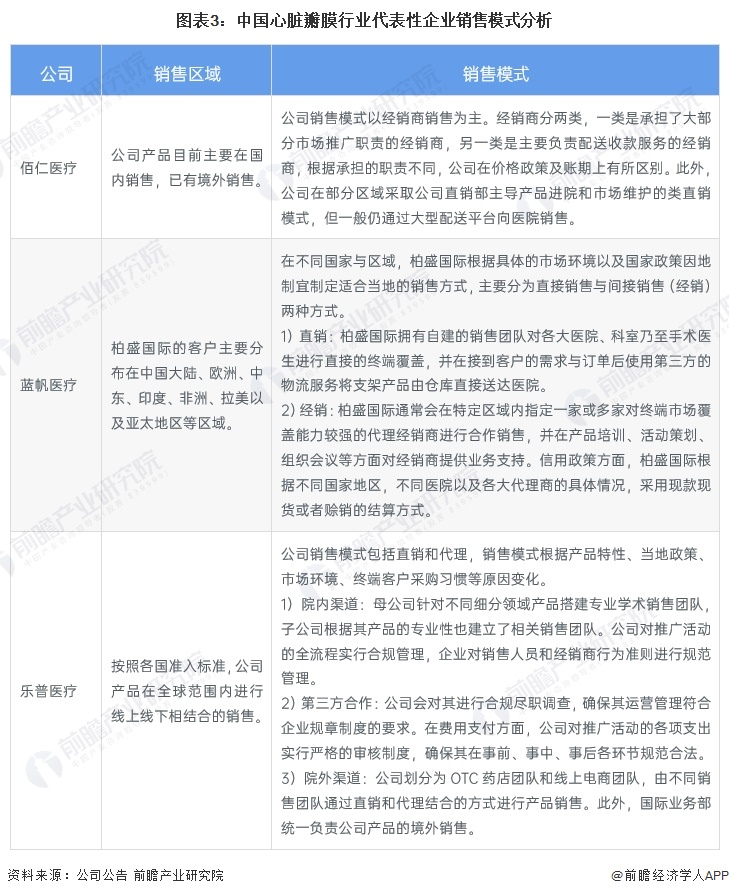
The distributor model is the main sales model for the heart valve industry
Bairen Medical, Blue Sail Medical and Lepu Medical are the leading enterprises in the heart valve industry in China. From the analysis of sales mode, Bairen Medical mainly sells to dealers. In addition, in some regions, the company adopts a quasi-direct sales model in which the company's direct sales department leads product admission and market maintenance, but generally still sells to hospitals through large distribution platforms.
The cardiovascular and cerebrovascular business division of Blue Sail Medical is mainly operated by its subsidiary Balson International. In different countries and regions, according to the specific market environment and national policies to develop suitable local sales methods, mainly divided into direct sales and indirect sales (distribution) two ways.
Lepu medical sales model includes direct sales and agents, sales model according to product characteristics, local policies, market environment, end customer purchasing habits and other reasons to change. In terms of in-hospital channels, the parent company sets up professional academic sales teams for products in different segments, and the subsidiaries also set up relevant sales teams according to the professionalism of their products. In terms of third-party cooperation, the company will conduct compliance due diligence on them to ensure that their operation and management meet the requirements of corporate rules and regulations; In terms of out-of-hospital channels, the company is divided into OTC pharmacy team and online e-commerce team, and different sales teams sell products through a combination of direct sales and agency. In addition, the International Business Department is responsible for the overseas sales of the company's products.
Comparison of product layout of Chinese heart valve industry enterprises
In summary, Qiming Medical is the company with the widest coverage of heart valve products and solutions in China, and has corresponding products in the fields of aortic, pulmonary, mitral and tricuspid valve diseases. In contrast, other companies are focused on the development of a subdivision of valve disease replacement or repair solutions, Peijia Medical, Bai Ren Medical is expanding into other areas of business, but the product is still in the design stage.

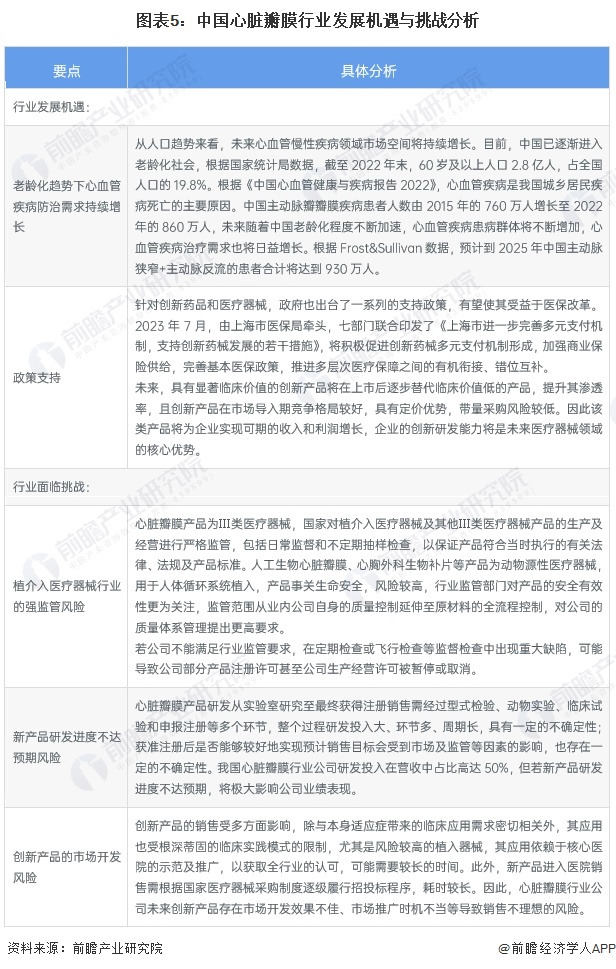
China's heart valve industry development opportunities and challenges
The development opportunities of China's heart valve industry include: the demand for cardiovascular disease prevention and control continues to grow under the aging trend, and industry policies promote technological innovation.
The challenges facing the heart valve industry in China are mainly: the strong regulatory risk of the implant intervention medical device industry, the risk of new product research and development progress is not up to expectations, and the market development risk of innovative products. The medical device industry has high requirements for technological innovation and product research and development capabilities, and the research and development cycle is long. The R&D investment of China's heart valve industry companies accounts for as much as 50% of the revenue, but if the progress of new product research and development does not meet expectations, it will greatly affect the company's performance. Taking Bairen Medical as an example, its existing products in the field of animal-derived implant interventional medical devices still have a large room for improvement from the ideal solution, and the company needs to continue to improve existing products and constantly develop new products.
In addition, the commercialization risk of products in research is also a challenge faced by enterprises in the industry. Take Lanfan Technology as an example, the company is currently developing a number of heavy products including a new generation of transcatheter aortic valve replacement system, coronary blood duct stone balloon dilatation catheter, coronary artery notch balloon dilatation catheter, transcatheter mitral valve membrane repair system, etc., which need to go through design verification, registration testing, clinical trials, registration approval and other stages. Product registration certificates issued by relevant regulatory agencies at home and abroad can only be marketed. Failure to achieve market acceptance in the physician, end-hospital, patient and medical fields for products that are not yet on the market will adversely affect the successful commercialization of such products and the achievement of economic benefits of a certain scale.
For more research and analysis of this industry, please refer to the "China Cardiovascular Access Device Industry Market Foresight and Investment Strategic Planning Analysis Report" of Prospective Industry Research Institute.
|
Last:Reprint: Market segment analysis of China ophthalmic high-value consumables industry in 2024
Next:Appearance and function examination of surgical instruments |
Return |