Core data of this paper: competition pattern, product layout, Porter's five forces, etc
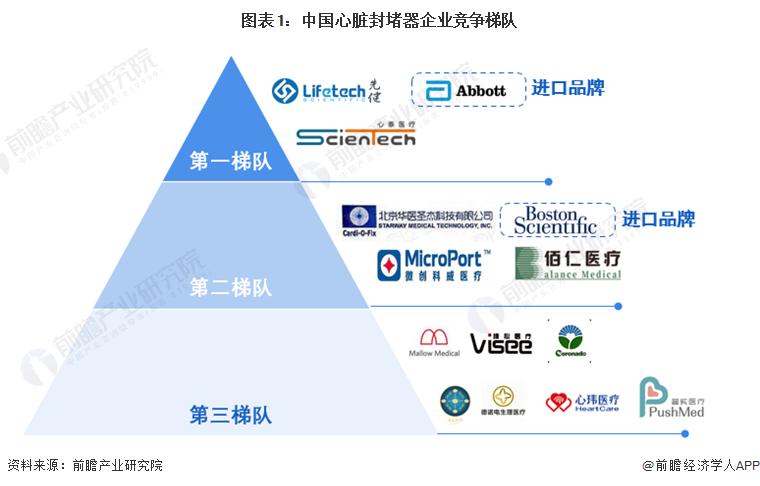
1, China's heart occluder industry competition echelon
China's heart occluder industry can be divided into three main competitive echelons: the first echelon is domestic brands such as Xintai Medical, Xiankin Technology and imported brands such as Abbott, the heart occluder business layout is relatively perfect, occupying the main domestic market share; The second tier is the domestic brands represented by Coldwell Medical, Huashingjie and Bairen Medical, as well as the imported brands such as Boston Scientific, which occupy a certain market share in some segments; The third echelon is other domestic local manufacturing enterprises, such as Jinkui Medical, Pulse Medical, Xinwei Medical, Pushi Medical, etc., which entered the industry relatively late and has a low market share.
2, China's heart occluder industry product layout
-- Breadth of layout: Xintai Medical's five types of heart occluder products have been commercialized
In China, there are more enterprises with the products of atrial septal defect closure, ventricular septal defect closure, patent ductus arteriosus closure and left atrial ear closure, and fewer enterprises with the products of patent ovale closure. From the perspective of the breadth of enterprise layout, the layout of cardiac occluders of Xintai Medical is relatively perfect, and the atrial septal defect occluders, ventricular septal defect occluders, patent ductus arteriosus occluders, patent foramen ovale occluders and left atrial ear occluders have been commercialized. First health technology, Chinese medical Shengjie, Pushi medical layout is relatively perfect.
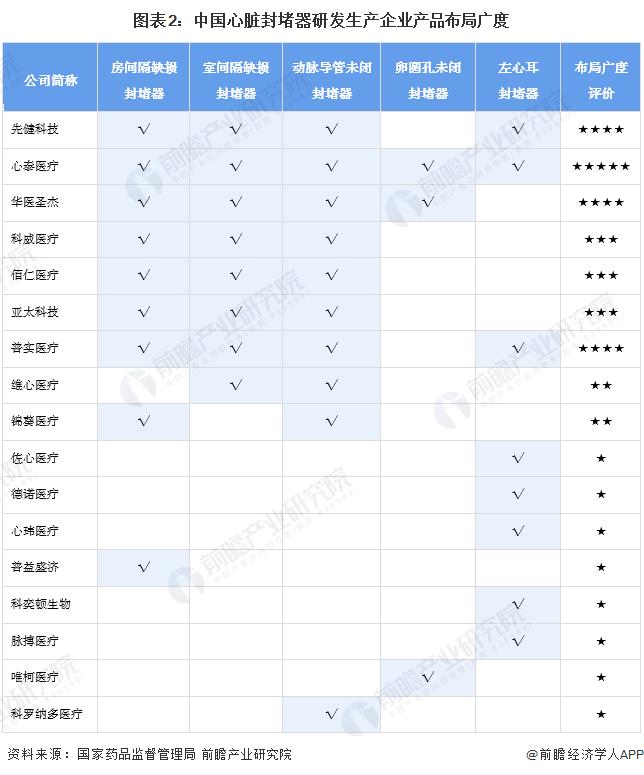
注:依據(jù)獲得國家藥品監(jiān)督管理局頒布的心臟封堵器產(chǎn)品注冊證產(chǎn)品類型劃分;2)布局廣度評價滿分為5星;3)包含歷史注冊證及有效注冊證;4)“√”表示該企業(yè)擁有該類產(chǎn)品注冊證
-- Layout strength: there are a large number of registration certificates of Xianjian Technology heart occluder products
In China, the number of registration certificates of atrial septal defect occluders, ventricular septal defect occluders and patent ductus arteriosus occluders is more, but the number of registration certificates of left atrial ear occluders and patent foramen ovale occluders is less. From the point of view of enterprise layout strength, the number of heart occluder product registration certificates obtained by First Health Technology is larger, followed by Xintai Medical, Hua Medical Shengjie, Kowei Medical.
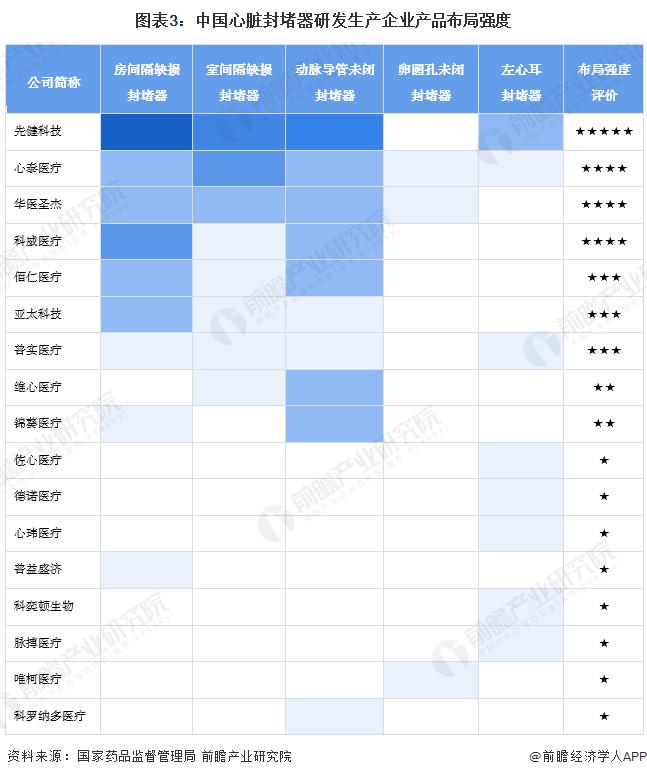
注:依據(jù)獲得國家藥品監(jiān)督管理局頒布的心臟封堵器產(chǎn)品注冊證產(chǎn)品類型劃分;2)布局強(qiáng)度評價滿分為5星;3)包含歷史注冊證及有效注冊證;4)顏色越深表示相關(guān)產(chǎn)品注冊證數(shù)量越多
3. Competition pattern of China's heart occluder industry
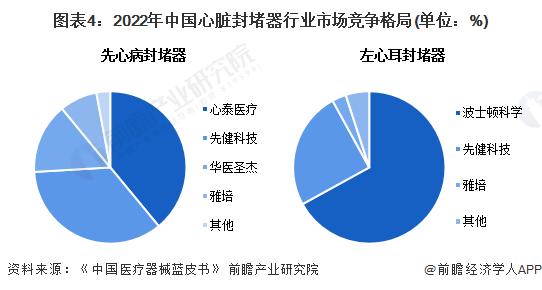
-- Market situation: Domestic enterprises occupy the main market in the field of congenital heart disease occluder
The domestic congenital heart disease occluder market is highly concentrated, showing a three-pronged position, Xintai Medical, Xianjian technology, Hua Medical Shengjie occupy about 90% of the market share; In the market of cardiogenic stroke occluder, imported enterprises occupy the main market share. In the field of left atrial appendage occluder, the imported brand Boston Scientific occupies 67% market share, and the domestic brand First Health Technology occupies 25% market share.
-- Registration: Xianjian Technology has a big advantage in the field of congenital heart disease occluder
From the perspective of obtaining the registration certificate of cardiac occluder products, as of February 2024, the State Drug Administration has issued a total of 85 registration certificates of cardiac occluder products, of which the number of registration certificates obtained by the leading domestic cardiovascular and cerebrovascular interventional medical device company Xianhealth Technology is the largest, with a total of 17; Abbott, the international leader in medical devices, received the second largest number of registration certificates, reaching 16.
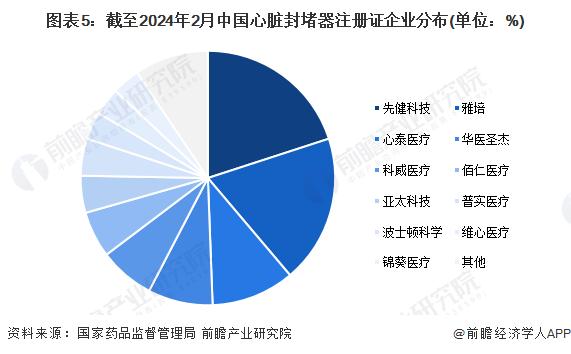
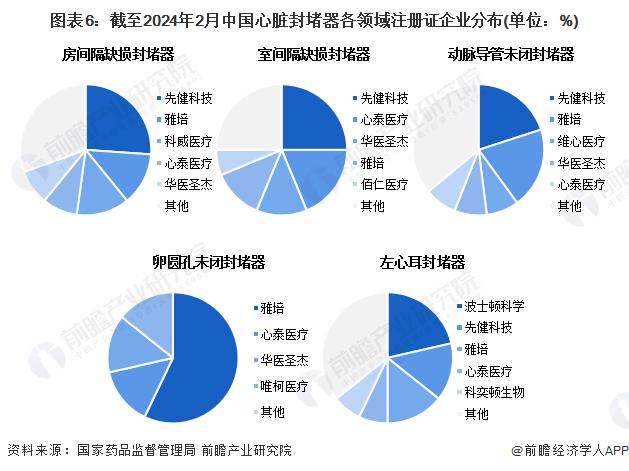
From the perspective of different segments, Xianjian technology occupies a greater advantage in the field of congenital heart disease occluder. In the field of atrial septal defect occlader, as of February 2024, the number of registration certificates obtained by Prehealth Technology was 6, followed by Abbott and Coldwell Medical; In the field of ventricular septal defect occluders, Xianjian Technology obtained a large number of registration certificates, 4, followed by Xintai Medical; In the field of patent ductus arteriosus occluders, the number of registration certificates obtained by Prehealth Technology and Abbott was 5, ranking first; In the field of patent foramen ovale occluders, imported brands occupy a greater advantage, Abbott obtained 4 registration certificates, accounting for more than 50%; In the field of left atrial appendage occlader, Boston Scientific has the largest number of registration certificates, with 3, followed by First Health Technology and Abbott, both with 2.
4, China's heart occluder industry market concentration
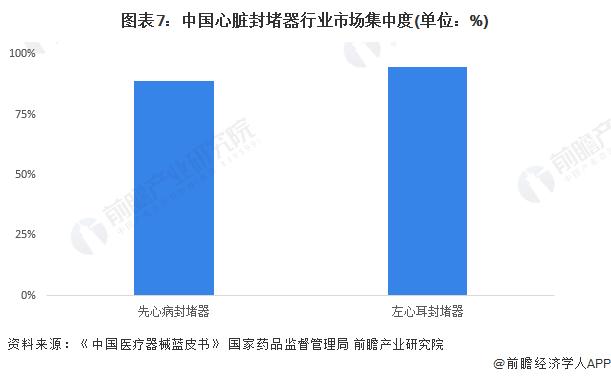
-- Market conditions: Market concentration is at an extremely high level
The market concentration of cardiac occluder in China is relatively high, and the oligarchic competition pattern has basically formed, with Xinthai Medical, Prehealth Technology, Kower Medical and Boston Scientific occupying the main market share of cardiac occluder in China. In the field of congenital heart disease occluder, CR3 is about 90%; In the field of left atrial appendage occluder, CR3 is about 95%.
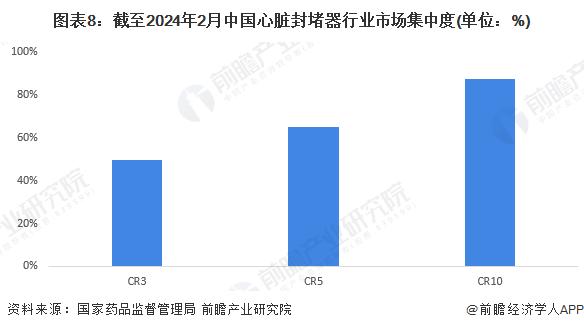
-- Registration situation: Patent foramen ovale sealer product registration certificate distribution is relatively concentrated
From the perspective of registration, the distribution of heart occluder product registration certificate enterprises in China is relatively dispersed, and the number of heart occluder product registration certificates owned by CR3 enterprises accounts for 50%; The number of registration certificates of heart occluder products owned by CR10 enterprises accounted for 87%.
注:依據(jù)注冊證數(shù)量劃分
From the market concentration of each segment, the product registration certificate distribution of patent foramen ovale occlader is relatively concentrated, CR3 is more than 85%; The distribution of registration certificates of atrial septal defect occluders, ventricular septal defect occluders, patent ductus arteriosus occluders and left atrial ear occluders was relatively dispersed, and CR3 was about 50%.
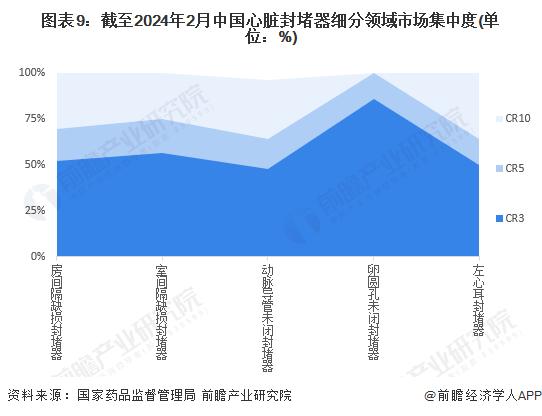
注:依據(jù)注冊證數(shù)量劃分
5. Summary of competition in China's heart occluder industry
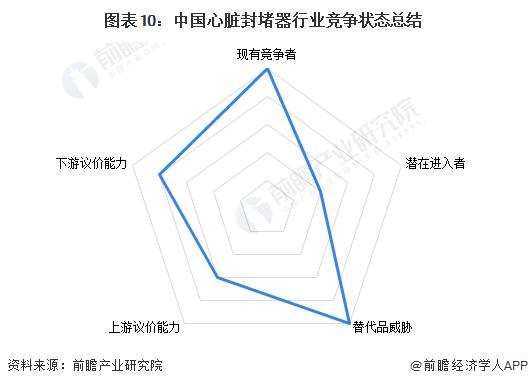
From the perspective of five-force competition model, from the competition situation, China's heart occluder market concentration is high, oligopoly competition pattern is basically formed, and the market competition is relatively fierce; From the perspective of potential entrants' threats, the higher capital investment, higher technical level requirements and higher entry barriers in the process of R & D and manufacturing of cardiac occluders; From the perspective of alternative threat, heart occluder products are irreplaceable for patients with congenital heart disease and cardiogenic stroke, and the alternative threat is small. From the perspective of upstream bargaining power, the upstream of the heart occluder industry mainly includes medical metal materials such as nickel-titanium alloy and degradable bio-polymer materials such as polylactic acid and polyurethane. Although the number of upstream participating enterprises is relatively large, the requirements for materials and technical level are high, and the upstream participants have a certain right to say; From the perspective of downstream bargaining power, the downstream mainly includes medical device dealers, medical institutions, congenital heart disease and cardiogenic stroke patients. At present, the number of cardiac occluder R & D and production enterprises is small, and the space for medical institutions to choose is small, and the bargaining power of downstream sales terminals is weak.
Based on the above analysis, the competition status of China's heart occluder industry is summarized as follows:
|
Last:Reprint: Market segment analysis of China ophthalmic high-value consumables industry in 2024
Next:Appearance and function examination of surgical instruments |
Return |