Core data of this paper: the number of product registrations
Overall situation: The number of products entering the medical consumables classification catalog system is nearly 1 billion
-- The number of registered domestic products accounted for 87 percent
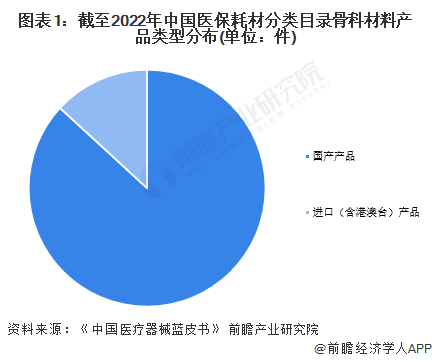
By the end of 2022, according to the information of the State Medical Insurance Bureau, there were 99,933 products in the national orthopedic materials into the medical insurance consumables classification and catalog system, and 13,156 products were imported (including Hong Kong, Macao and Taiwan), accounting for 13%; The number of domestic products was 86,777, accounting for 87%.
-- Imported products mainly come from the United States, Switzerland and other places
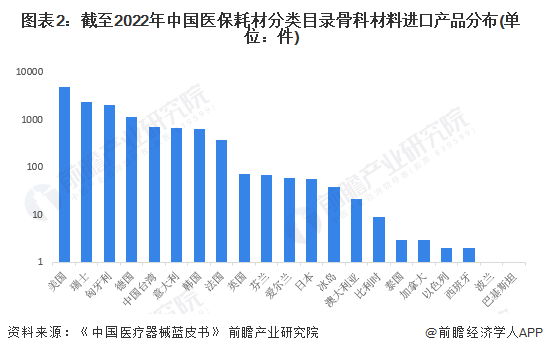
Imported products are mainly from the United States, Switzerland, Hungary and Germany. Among them, the number of orthopedic material products included in China's medical consumables classification catalog in the United States reached 4,850, covering 49 fields such as locking bone plates, interbody fusion devices, and titanium locking bone plate systems. The number of orthopedic material products included in the medical consumables classification catalogue in Switzerland reached 2358 pieces, covering 32 fields.
Regional distribution: The number of registered enterprises and products in Jiangsu ranks first in the country
-- Registered enterprises are concentrated in Jiangsu, Hebei and other places
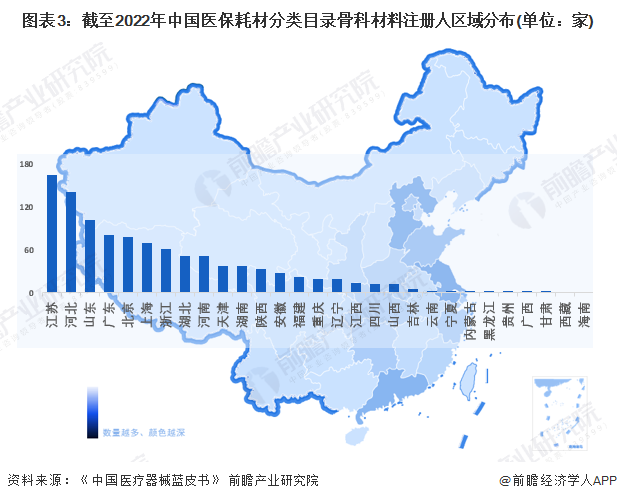
From the distribution of registered enterprises, at present, China's orthopedic implant medical device enterprises are mainly concentrated in Jiangsu, Hebei, Shandong and other places. According to the information of the State Medical Insurance Bureau, there are a total of 1057 registrants of orthopedic materials entering the medical insurance consumables classification directory in China, of which 164 are registered in Jiangsu Province, ranking first in the country; Hebei Province and Shandong Province ranked second and third with 141 and 101, respectively.
注:截至2022年底
-- There are more registered products in Jiangsu and Beijing
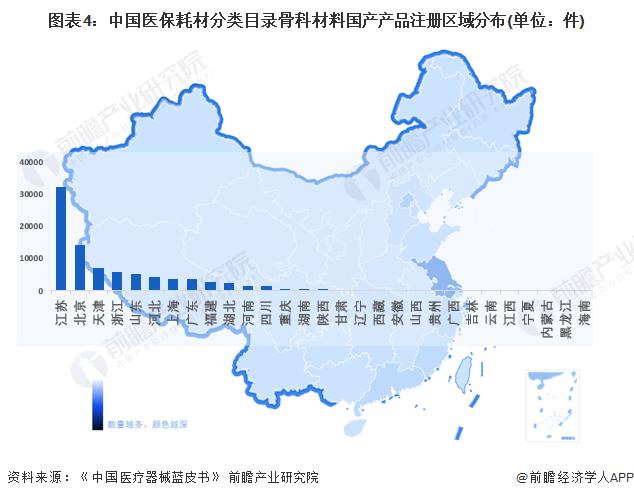
In China's medical consumables classification catalog, orthopedic domestic products are mainly concentrated in Jiangsu, Beijing and other places, by the end of 2022, the number of products are 32086, 14296.
注:截至2022年底
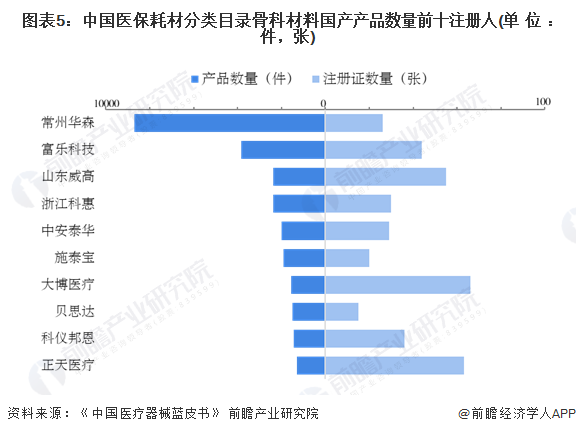
Enterprise competition: Dabo has the largest number of medical product registration certificates, and Huasen has the first number of medical products
From the perspective of registrants, by the end of 2022, Dabo Medical has obtained the largest number of medical consumables - orthopedic domestic product registration certificates, totaling 66; Changzhou Huasen Medical products have the largest number, with a total of 8,705 pieces.
注:截至2022年底
|
Last:Reprint: Market segment analysis of China ophthalmic high-value consumables industry in 2024
Next:Appearance and function examination of surgical instruments |
Return |