Comprehensive comparison of listed companies in China's heart valve industry in 2024 (with business layout summary, performance comparison, business planning, etc.)
Main listed companies in heart valve industry: Bai Ren Medical (688198.SH), Blue Sail Medical (002382.SZ), Lepu Medical (300003.SZ), Xintai Medical (02291.HK), Qiming Medical (02500.HK), Pejia Medical (09996.HK), Jianshi Technology (09877.HK), Microinvasive Medical (00853.H) K), Heart Medical (02160.HK), etc
Core data: Regional competition of Chinese heart valve enterprises; Comparison of business layout of Chinese heart valve enterprises; Comparison of business planning of Chinese heart valve enterprises
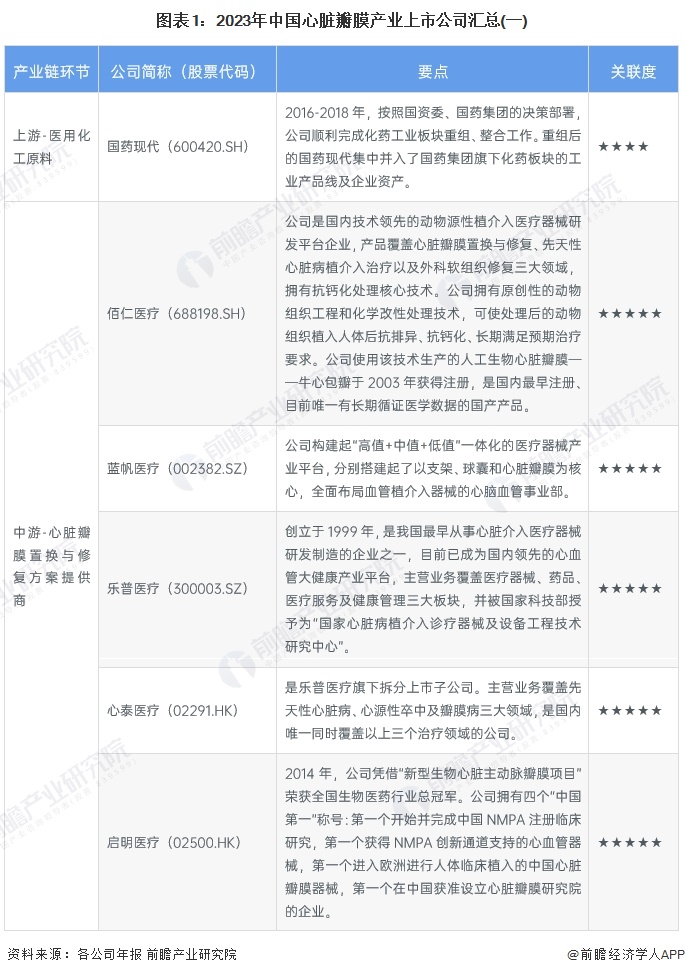
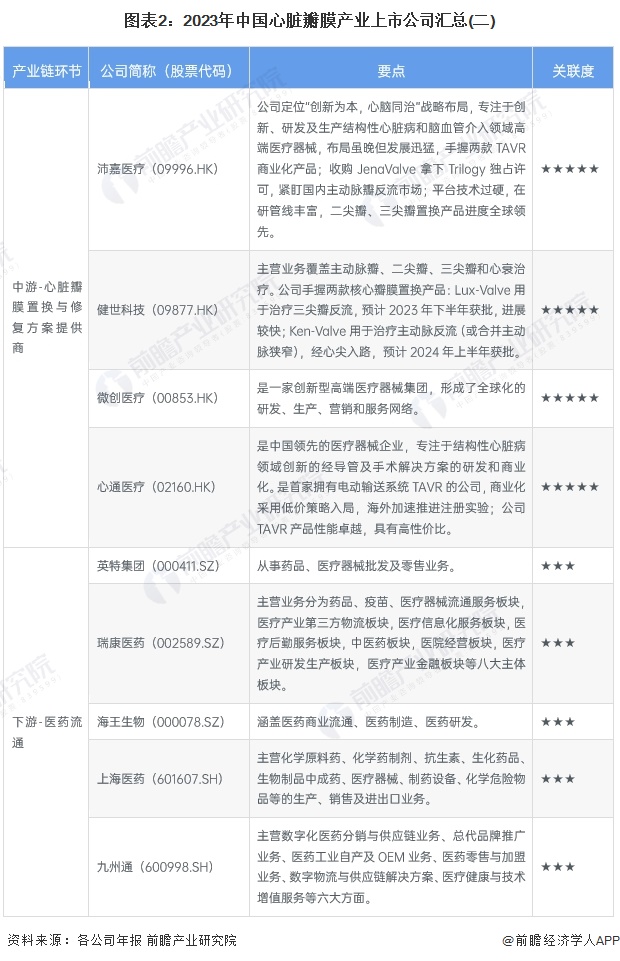
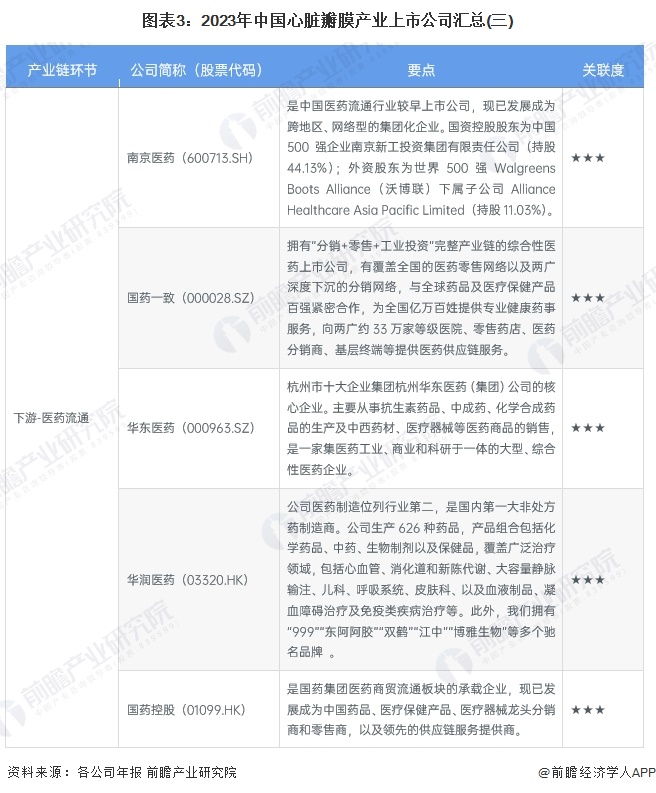
Cardiac valve industry listed companies summary
At present, the number of listed companies in China's heart valve industry is large. The upstream supplier of medical chemical raw materials is Sinopharm Modern (600420.SH); Middle heart valve replacement and repair solution providers include Bairen Medical (688198.SH), Blue Sail Medical (002382.SZ), Lepu Medical (300003.SZ), Xintai Medical (02291.HK), Qming Medical (02500.HK), Pejia Medical (09996.HK), Jianshi Technology (09877) .HK), Minimally Invasive Medical (00853.HK), Heart Medical (02160.HK); Downstream pharmaceutical distribution enterprises include Intertek Group (000411.SZ), Ruikang Pharmaceutical (002589.SZ), Haiwang Biological (000078.SZ), Shanghai Pharmaceutical (601607.SH), Kyushutong (600998.SH), Nanjing Pharmaceutical (600713.SH), Sinoparming (000028.SZ) ), East China Pharmaceutical (000963.SZ), China Resources Pharmaceutical (03320.HK), Sinopharmology Holdings (01099.HK).
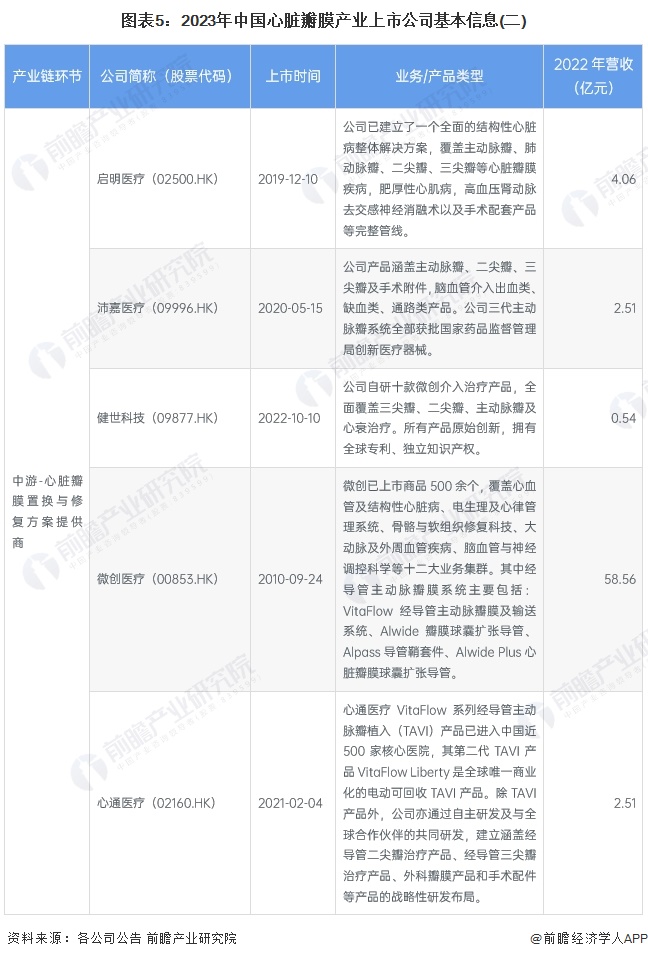
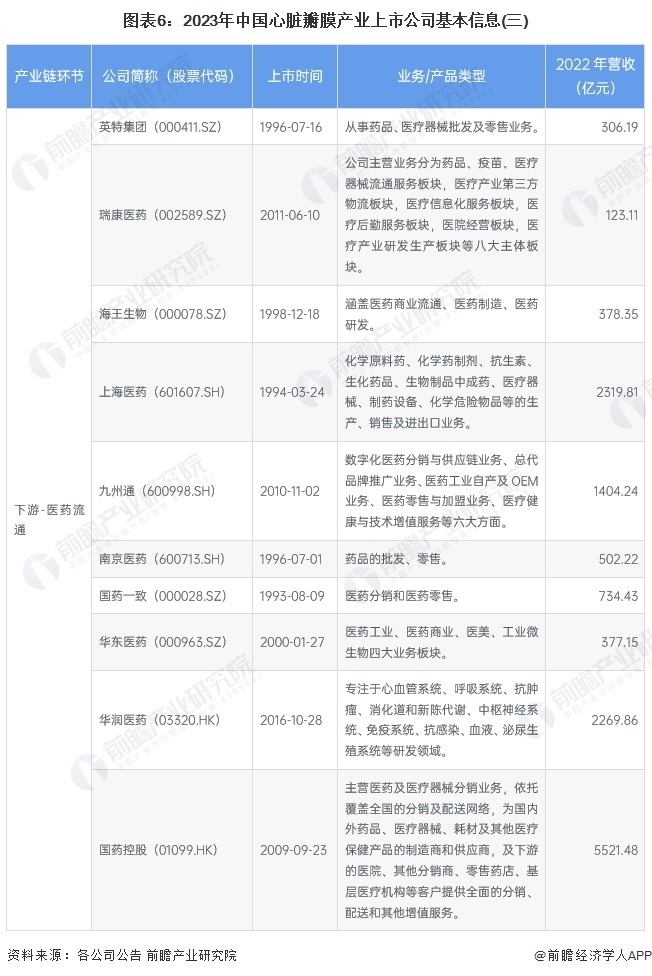
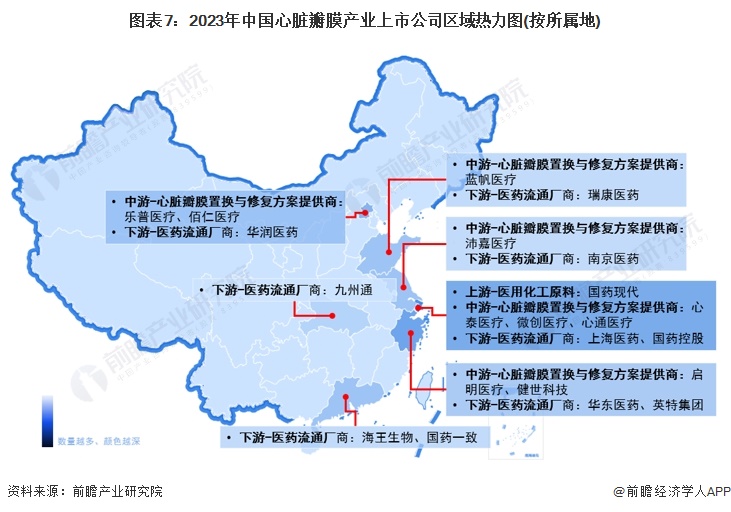
Comparison of business layout of listed companies in heart valve industry
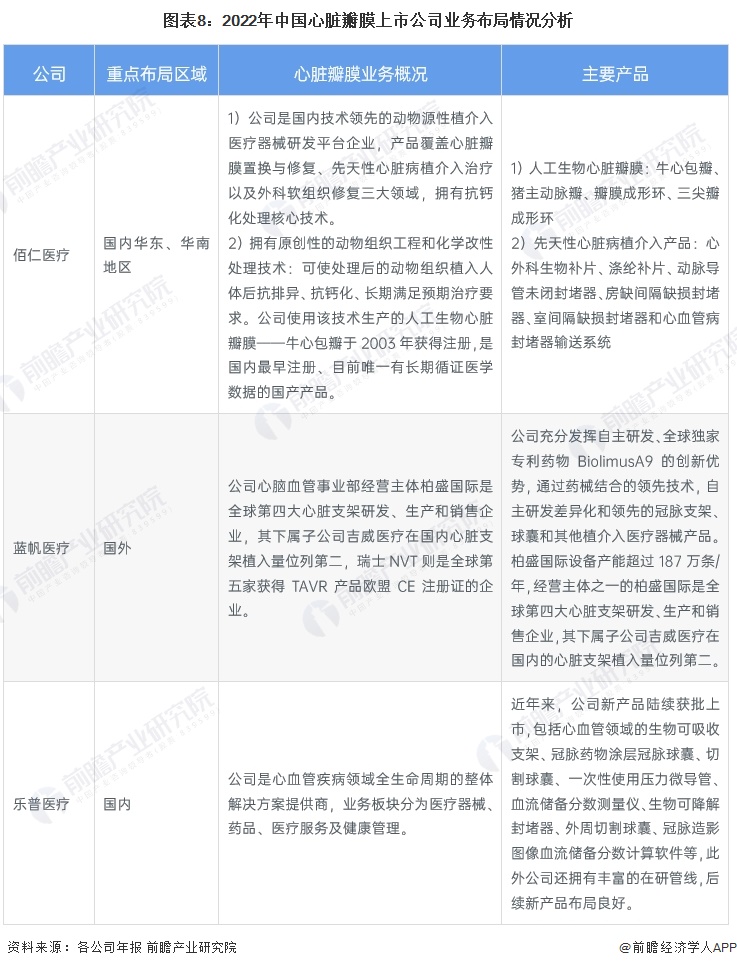
From the perspective of the business layout of listed companies in the heart valve industry in China, Bairen Medical is deeply engaged in the heart valve industry, and is a domestic technology leading animal-derived implantation interventional medical device R & D platform enterprise, with products covering three major fields of heart valve replacement and repair, congenital heart disease implantation interventional treatment and surgical soft tissue repair.
Companies such as Blue Sail Medical and Lepu Medical use the heart valve business as part of the group's diversification in the large medical industry. Lepu Medical is a full life cycle solution provider in the field of cardiovascular diseases, with business segments of medical devices, pharmaceuticals, medical services and health management. The company has maintained its industry position and competitive advantage as a long-term leader in cardiovascular implantation intervention in China, and has developed and commercialized several "first in China", including coronary stents, cardiac pacemakers, coronary bioabsorbable stents, coronary cutting balloons, and biodegradable occlusions. Based on the "A+X" development strategy, along the "high, medium and low value" multiple medical device consumables track, Blue Sail Medical has developed into a multinational medical device integrated platform enterprise with China as the core and facing the world.
Comparison of performance of listed companies in the heart valve industry
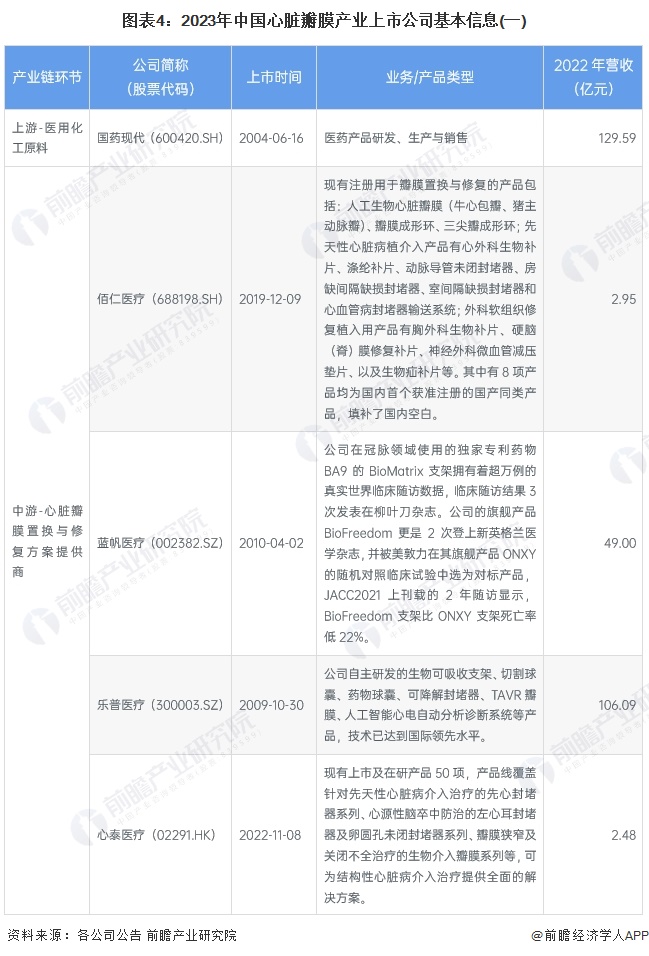
From the perspective of the income of listed companies in the heart valve industry, Lepu Medical income is higher, more than 10 billion yuan. The company is deeply engaged in innovative cardiovascular medical devices, has mature commercial products and a wealth of research pipelines in the field of coronary artery and structural heart disease, and constantly promotes the research and development of peripheral vascular, heart rhythm management, electrophysiology, neuroregulation, heart failure and other fields.
Minimally invasive medical, Blue Sail medical revenue is also much higher than other enterprises, respectively, 4.9 billion yuan and 5.9 billion yuan. Among them, Berson International, the operating body of the cardio and cerebrovascular division of Blue Sail Medical, is the world's fourth largest heart stent R&D, production and sales enterprise, its subsidiary Jiwei Medical ranks second in the number of heart stent implants in China, and Switzerland NVT is the world's fifth enterprise to obtain the EU CE registration certificate of TAVR products.
From the perspective of gross profit rate of listed companies, Bairen Medical and Xintai Medical have the highest gross profit rate, close to 90%. Bairen Medical is leading in the research and development of heart valve treatment, and the company has completed the professional communication with the Medical device Technical Review Center of the State Food and Drug Administration and recently obtained the approval of registration, which is a key part of the product layout of valvular disease treatment.

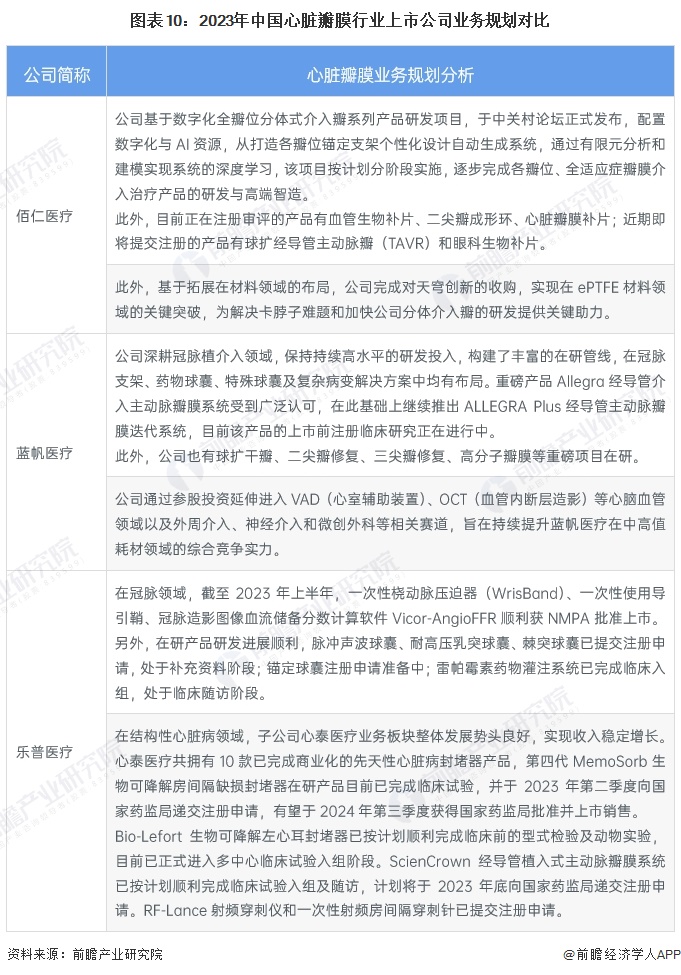
Comparison of business planning of listed companies in heart valve industry
A number of Bairen medical research products are progressing smoothly. As of the first half of 2023, the company has submitted registration applications for its main products, vascular biological patch, spherical expansion interventional aortic valve and interventional middle valve have entered the innovation channel, and the one-year follow-up after clinical trial has achieved good results. Among them, the interventional middle valve has successfully submitted registration applications, and the interventional aortic valve is about to complete pre-registration preparation. The clinical trial of interventional pulmonary valve has been completed and enrolled, the split interventional valve is progressing smoothly, and the animal test effect of split interventional aortic valve has reached the expectation. In addition, based on the expansion of the layout in the field of materials, the company completed the acquisition of Skydome Innovation to achieve key breakthroughs in the field of ePTFE materials, providing key help to solve the problem of jam neck and accelerate the development of the company's separate intervention valve.
Bluesail Medical innovation research has excellent leading technology advantages, its Heart valve products have been published in the European Heart Journal, JACC(Journal of the American College of Cardiology) and other papers, with 26 literature, 8 post-marketing clinical trials. As the first Mox drug-coated balloon approved by the special review of innovative medical devices in China, Berton BA9DCB has filled the gap in the application of rapamycin and its derivatives in the field of drug balloons, providing a safer and more effective treatment plan for the treatment of coronary small vessel diseases. In addition, the company extends into the cardiovascular and cerebrovascular fields such as VAD(ventricular assist device), OCT(intravascular tomography) and other related tracks such as peripheral intervention, neurological intervention and minimally invasive surgery through equity investment, aiming to continue to improve the comprehensive competitive strength of Blue Sails Medical in the field of mid-to-high value consumables.
Lepu Medical's innovative portfolio of coronary implantation interventional products has led and promoted the treatment of coronary heart disease in China into a new era of "intervention-free implantation" and achieved outstanding commercial success. The company's products have experienced long-term large-scale clinical application in the real world, the quality system is widely recognized by doctors and patients, and the brand recognition is strong, forming the company's core competitiveness in the field of cardiovascular implantation intervention. The company has laid out a series of products under research in the field of cardiovascular medical devices, which are expected to achieve commercial sales in 3-5 years, and the listing of new products will bring new sources of revenue for the company and drive future performance growth.
For more research and analysis of this industry, please refer to the "China Cardiovascular Access Device Industry Market Foresight and Investment Strategic Planning Analysis Report" of Prospective Industry Research Institute.
|
Last:Reprint: Market segment analysis of China ophthalmic high-value consumables industry in 2024
Next:Appearance and function examination of surgical instruments |
Return |