
01
General situation
| 1. Product category
According to JOINCHAIN statistics, in the first half of 2024, the number of new medical device product registrations in the United States reached 1,618, an increase of 4.93%. A total of 1,138 companies (medical device manufacturers) in 59 countries were involved. Among them, 164 enterprises in China obtained 212 medical device product registration certificates in the United States, accounting for 13.10% of the total number of new registrations of medical device products in the United States, and the number of new product registrations decreased by 4.07% compared with the same period last year.
Figure 1 Registration certificate of medical device products in the United States from 2020 to 2024H1
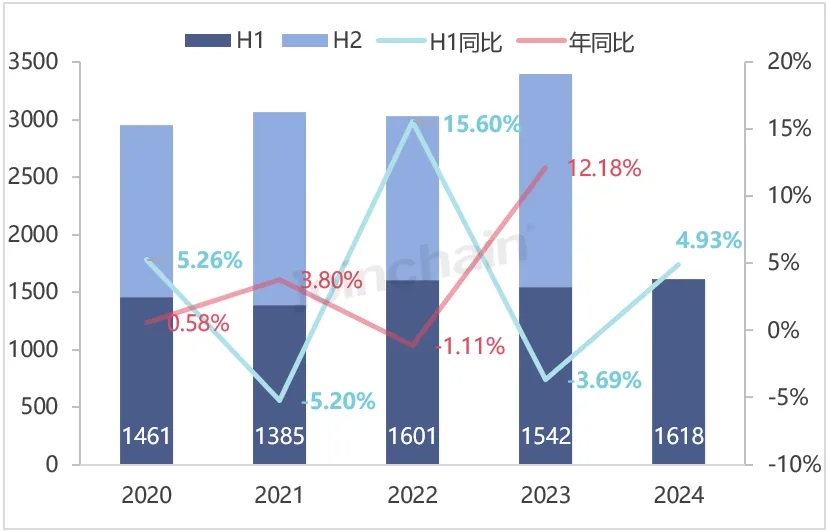
Data source: MDCLOUD (Medical Device Data Cloud)
Figure 2 Chinese enterprises obtaining registration certificates of medical devices in the United States from 2020 to 2024H1
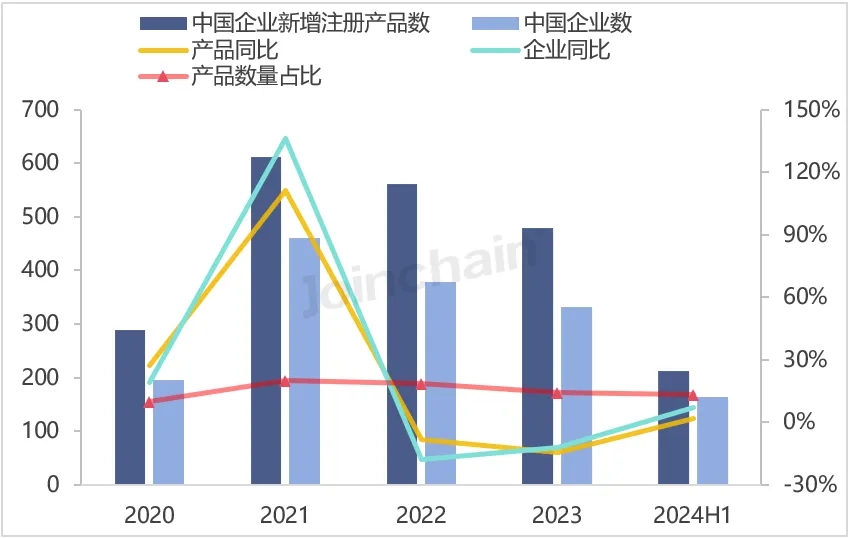
Data source: MDCLOUD (Medical Device Data Cloud)
| 2. Product category
According to JOINCHAIN statistics, the number of new medical device product registrations in Australia in the first half of 2024 reached 2,033, down 2.40% year-on-year. A total of 1023 companies (medical device manufacturers) in 52 countries were involved. Among them, 254 enterprises in China obtained 427 medical device product registration certificates in Australia, accounting for 21.00% of the total number of new medical device products registered in Australia, and the number of products increased by 21.31% year-on-year.
Figure 3 The registration certificate of medical device products in Australia from 2020 to 2024H1
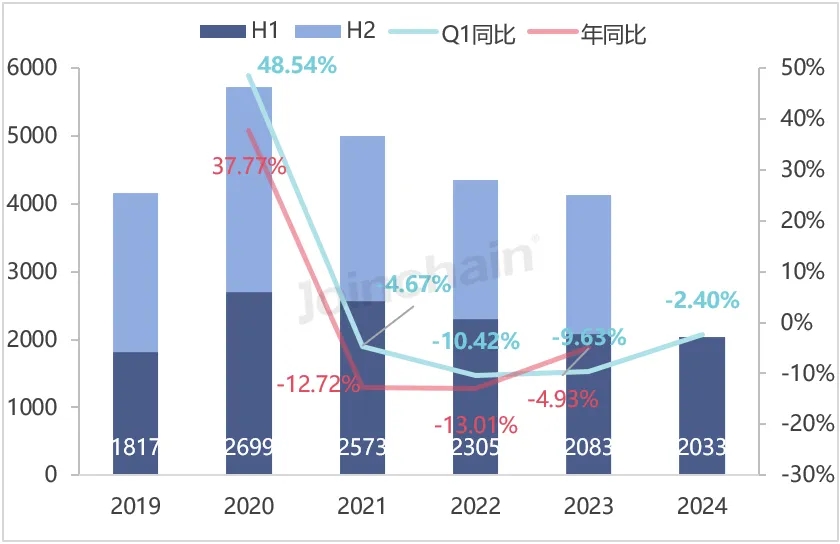
Data source: MDCLOUD (Medical Device Data Cloud)
Figure 4. 2020-2024H1 Chinese enterprises obtaining registration certificates for medical devices in Australia
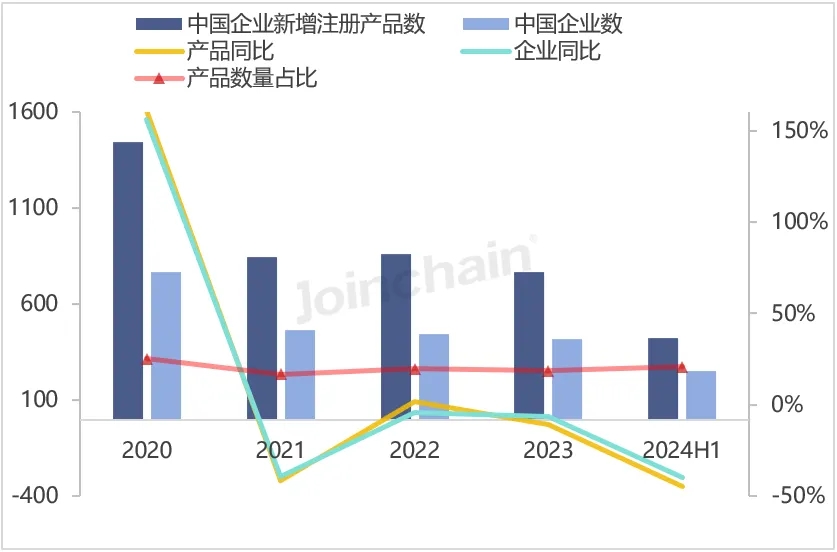
Data source: MDCLOUD (Medical Device Data Cloud)
02
Regional analysis
| 1. The United States
According to JOINCHAIN statistics, in the first half of 2024, among the countries that obtained the registration certificate of medical device products in the United States, the United States ranked first with 763 products, accounting for 47.16%, involving 507 enterprises; China (excluding Hong Kong, Macao and Taiwan) ranked second, with 212 products, accounting for 13.10%, involving 164 enterprises; Ranked third is South Korea, the number of newly registered products is 62, accounting for 3.83%, involving 49 companies.
Figure 5 Distribution of newly registered products in the United States in 2024H1 (Top10)
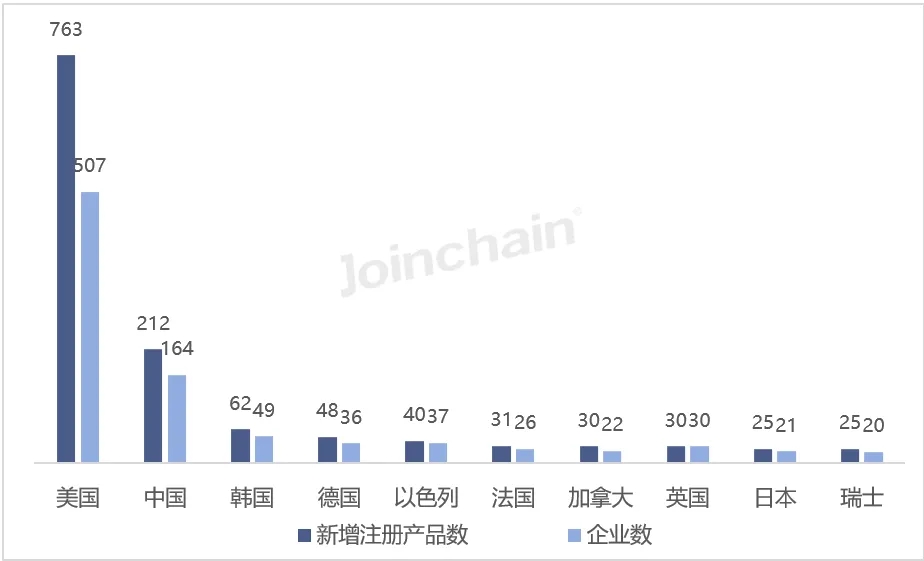
Data source: MDCLOUD (Medical Device Data Cloud)
From the distribution of enterprises in provinces, enterprises in Guangdong Province obtained the largest number of products, 81, accounting for 38.21%, involving 59 enterprises; Ranked second and third are Jiangsu Province and Zhejiang Province, the number of products are 30 and 20, accounting for 14.15% and 9.43%, respectively, and the number of enterprises involved are 25 and 17 respectively.
Table 1 Province distribution of newly registered products of Chinese enterprises in 2024H1 United States
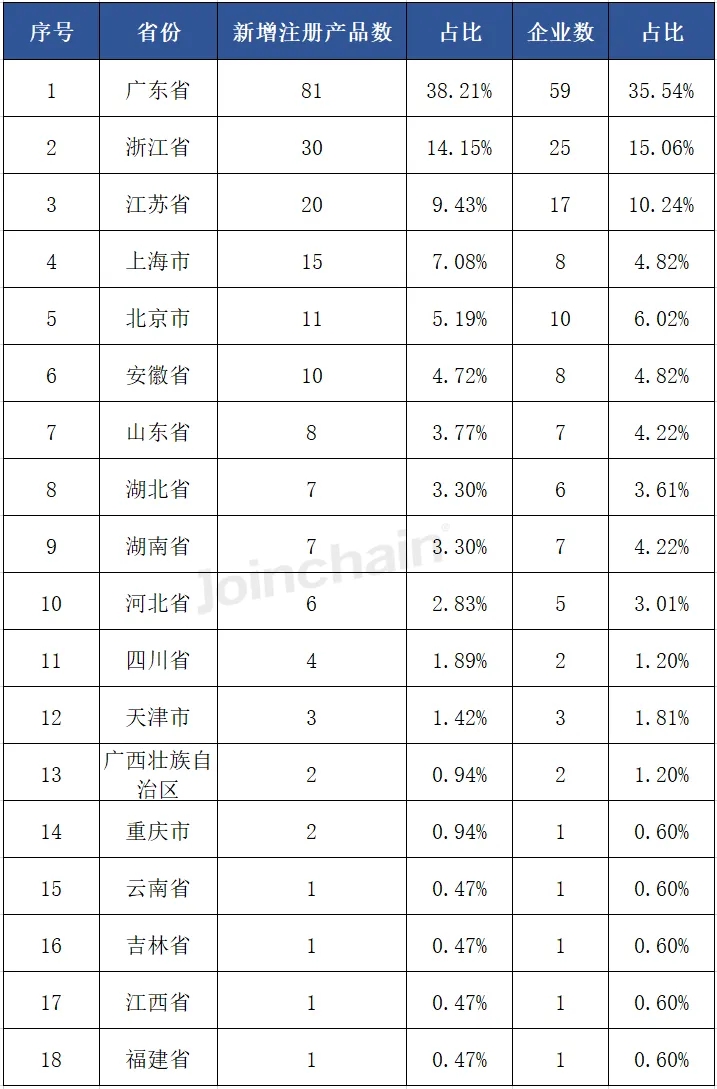
Data source: MDCLOUD (Medical Device Data Cloud)
| 2. Australia
According to JOINCHAIN statistics, in the first half of 2024, among the countries that obtained the registration certificate of medical device products in Australia, the United States ranked first with 458 products, accounting for 22.53%, involving 174 enterprises; China (excluding Hong Kong, Macao and Taiwan) ranked second, with 427 products, accounting for 21.00%, involving 254 enterprises; Ranked third is Germany, the number of newly registered products is 272, accounting for 13.38%, involving 120 enterprises; Australia came in fourth.
Figure 6 Distribution of newly registered products in Australia in 2024H1 (Top10)
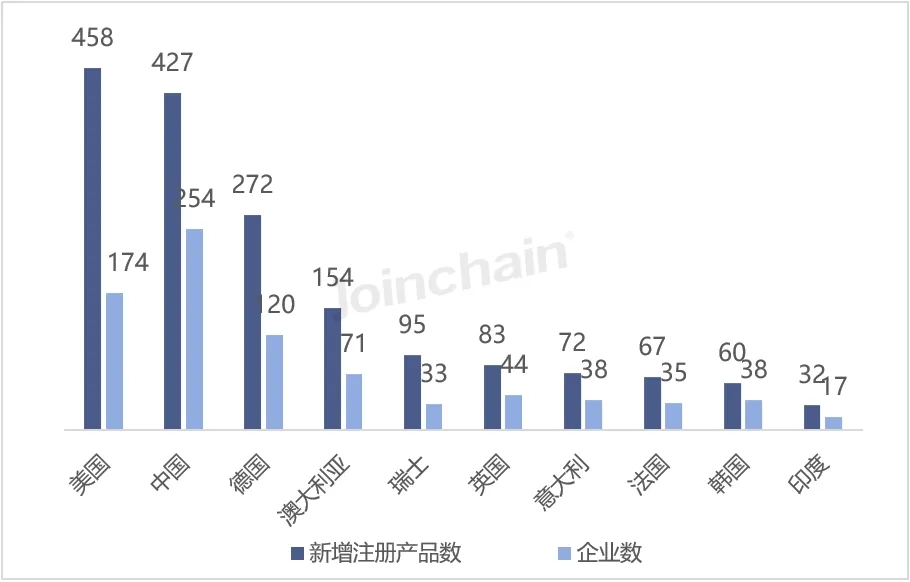
Data source: MDCLOUD (Medical Device Data Cloud)
From the distribution of enterprises in provinces, enterprises in Guangdong Province obtained the largest number of products, 139, accounting for 32.55%, involving 78 enterprises; Ranked second and third are Jiangsu Province and Zhejiang Province, the number of products were 82 and 78, accounting for 19.20% and 18.27% respectively, and the number of enterprises involved were 40 and 42 respectively.
Table 2 Province distribution of Chinese enterprises with newly registered products in Australia in 2024H1
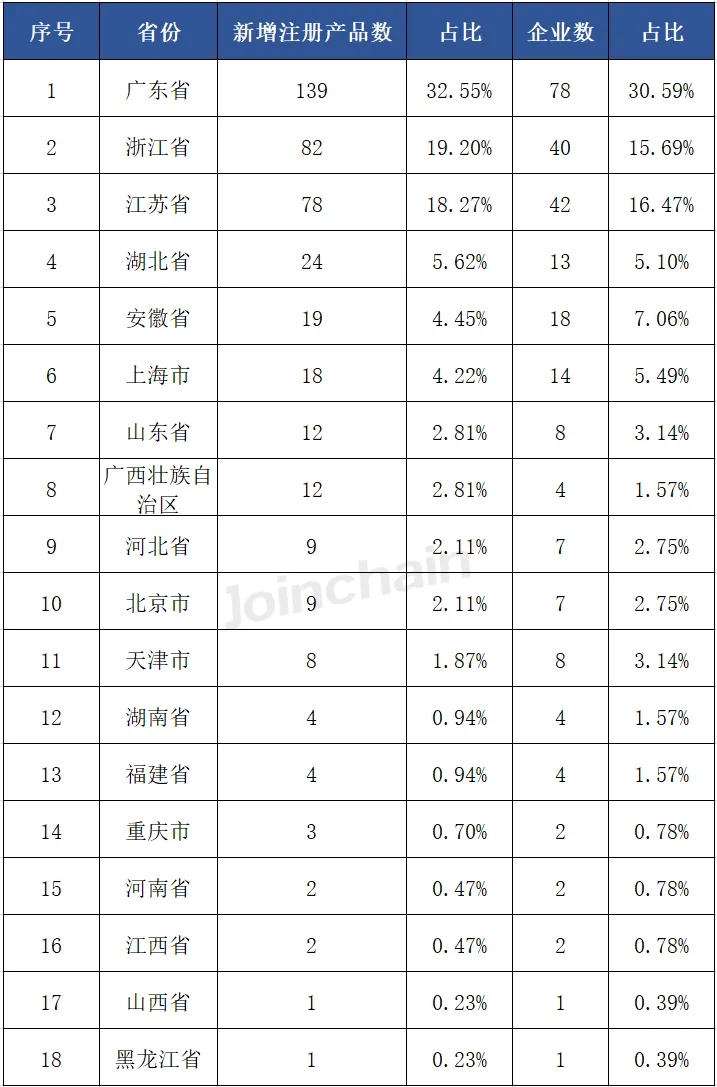
03
Corporate list
| 1. The United States
According to JOINCHAIN statistics, among the Chinese enterprises that obtained the registration certificate of medical device products in the United States in the first half of 2024, the top ones are Shanghai Lianying Medical Technology Co., LTD., Shenzhen Libang Precision Instrument Co., LTD., and Shenzhen Youlai Intelligent Electronics Co., LTD.
Figure 7 List of Chinese enterprises with newly registered products in the United States in 2024H1 (Top10)
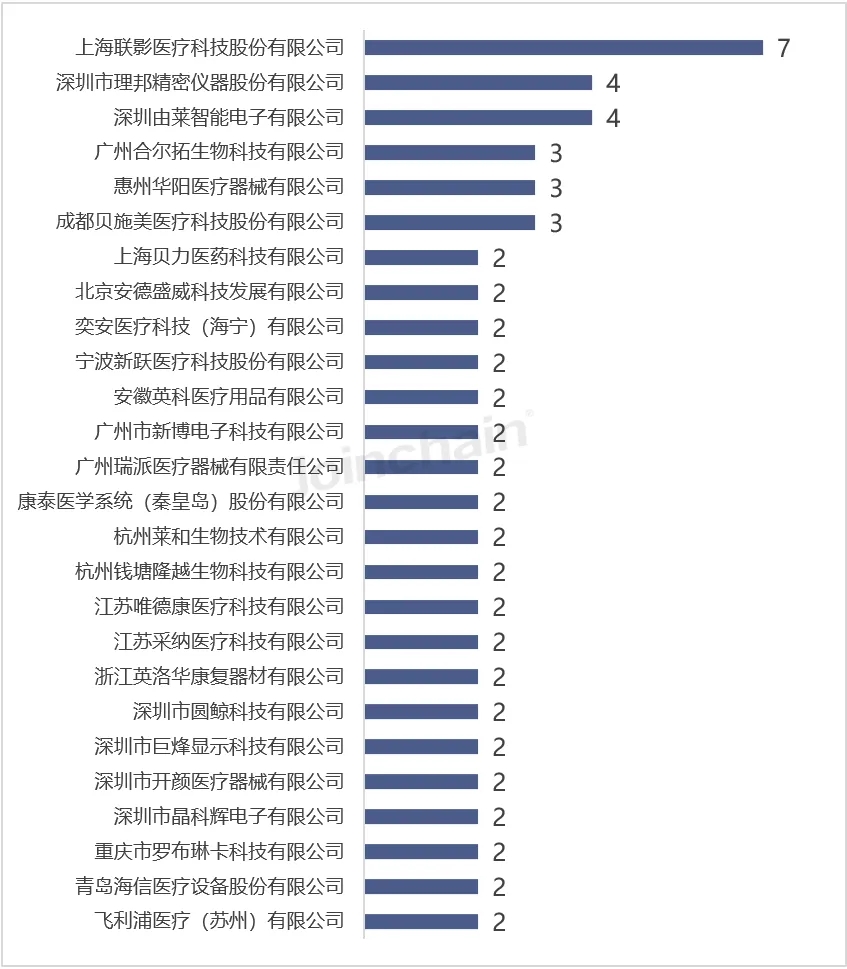
Data source: MDCLOUD (Medical Device Data Cloud)
| 2. Australia
According to JOINCHAIN statistics, among the Chinese enterprises that obtained the registration certificate of medical device products in Australia in the first half of 2024, Shiyuan Technology (Jiaxing) Medical Electronics Co., Ltd. ranked first in the number of product registrations, with 20 products; Followed by Changzhou Tongchuang Medical Device Technology Co., LTD., the number of products is 10; Guangzhou Weili Medical Equipment Co., Ltd. and Zhejiang Daewoo Pharmaceutical Co., Ltd. Jianshan Medical Store tied for third place, with 9 products.
Figure 7 List of Chinese enterprises with newly registered products in Australia in 2024H1 (Top10)
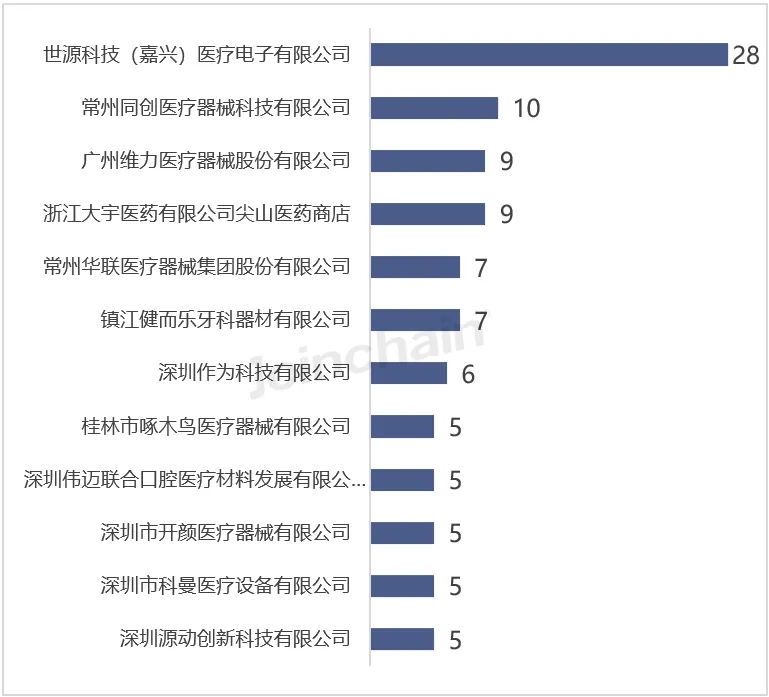
Data source: MDCLOUD (Medical Device Data Cloud)